News
Industrial
Different requirements for medical electronic sensors
Designers are facing a number of challenges in developing smaller, lighter, and more portable medical devices that save space in the ward and facilitate medical use. To meet the needs of global medical device manufacturers, sensor manufacturers must consider four key issues: miniaturization, material specification standards, supplier quality assurance, service and support.
In the medical device market, product features such as miniaturization, low power consumption and high liquid compatibility have become a global trend. However, in various countries and regions, material specification standards, product requirements and cost issues are not the same. This requires sensor manufacturers to offer a flexible, scalable product line to meet the needs of customers around the world. In addition, sensor manufacturers must ensure product quality and supply quality.
Miniaturization affects product size, portability and weight
Designers are facing a number of challenges in developing smaller, lighter, and more portable medical devices that save space in the ward and facilitate medical use. For example, traditional infusion pumps and insulin pumps are large in size, and after design optimization, the emergence of portable and portable devices has greatly benefited patients and healthcare professionals. For example, smaller sensors can be easily integrated into space-constrained infusion pumps. Due to its small size and low power consumption, the portability of the infusion pump is further enhanced, which helps to improve the patient's convenience. In addition, more and more patients choose to “treat at home” rather than “hospitalization” – a trend that is particularly prevalent in the US and EU countries. The transformation of treatment models has also driven the trend toward miniaturization of medical devices, including respiratory and diagnostic devices.
In some cases, miniaturization design will consider incorporating some diagnostic functions into a networked medical device to remotely monitor patient care and care. Therefore, it is necessary to use a sensor product that integrates multiple detection functions (such as humidity and temperature detection) in a single package. This will create possibilities for medical device manufacturers to reduce device size or increase device functionality. Respiratory devices for respiratory therapy, including ventilators and sleep ventilators, benefit from this design.
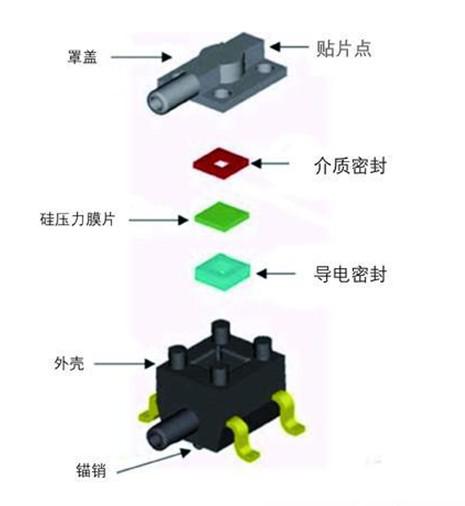
Figure 1: Modular design of the TruStability board-mounted pressure sensor from Honeywell Sensing and Control
Another way sensor manufacturers meet these requirements is to further develop the sensor product line, which offers a wide range of mechanical interfaces, mounting styles, packages and I/O options. This allows medical device designers to choose the right interface to reduce footprint, reduce costs and eliminate additional components. For example, pressure sensors should offer a variety of mounting options for installation in space-constrained applications, allowing designers to position sensors in the right place for accurate inspection. For applications that require precise measurements, sensor installation is critical. For example, in dialysis treatment, the accurate measurement of the pressure of the dialysate and vein using the sensor is directly related to the patient's life safety.
For portable applications, sensors need to meet both low voltage and low power requirements to extend battery life. The sensor should also provide both start-up and sleep modes, and the device consumes power only in the startup mode. The sensor's sleep mode extends battery life, reduces power supply size, and reduces the weight of medical equipment.
Material/specification standards affect global producers
For example, in an EU country, as long as there is little evidence that a material is harmful or dangerous, government regulators may include the material in the ban. Due to the sound medical system and sufficient funds in the EU countries, the medical device market is very large. Therefore, before the global medical device manufacturers enter the EU market, they must ensure that their products meet EU standards. Two regulations affecting the global electronics industry are the Directive on Restricting the Use of Certain Hazardous Substances in Electrical and Electronic Equipment (RoHS) and the Registration, Evaluation, Authorization and Restriction of Chemicals Act (REACH). The former aims to limit the six chemicals used in the electronics manufacturing industry in the past, while the latter provides the electronics industry with a framework for controlling the use of hazardous substances within the EU. RoHS and REACH affect the use of materials in end products and production processes. In order to sell products to EU countries, global manufacturers and distributors must comply with these regulations.
To ensure accurate measurement, the sensor must be as close as possible to the fluid (blood, infectious substances, salt solution, etc.) and in frequent contact with the patient's gas or liquid passages. Therefore, sensor manufacturers must ensure that selected materials, supply chains, and manufacturing processes comply with EU material specification standards.
In addition, EU regulations promote the use of RoHS-compliant liquid-media compatible sensors for a wide range of medical devices, from chemical analyzers to ventilators. To help medical device manufacturers address the “difficult” problem of cost, sensor manufacturers must further develop product lines that offer a wide range of output types, pressure types, pressure ranges, mounting options and package types to meet the needs of EU countries. .
Product platforms should offer a variety of options and components to meet the specific needs of different countries, and should help medical device design engineers meet the pricing of products from manufacturers and distributors around the world. In addition, it should allow engineers to adjust product flexibility based on application and country needs.
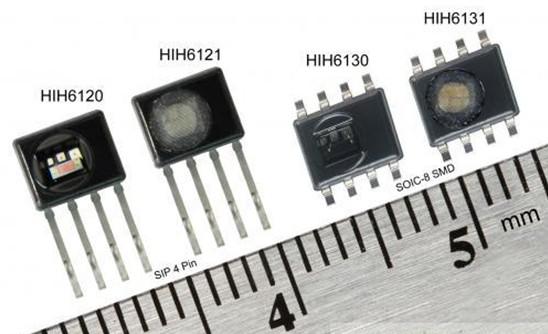
Figure 2: Honeywell's HumidIcon digital temperature and humidity sensor integrates two functions in a single small package that can operate at 2.3Vdc low voltage
For example, the design of respiratory instruments varies greatly across countries, and the requirements for package types vary. Flexible sensors are available in a variety of package options, both for integrated manifold designs in Western countries and for traditional manifold and interface designs common in developed countries.
Supplier quality assurance is the key
Sensor manufacturers must not only comply with US UL, Canadian cUL or CSA, European ENEC and China CQC and other material regulations and global electronic standards, but also develop quality assurance systems to ensure effective implementation of the regulatory process and control product quality.
The supplier's production process should meet Six Sigma quality standards to ensure the production of high quality products. Medical device products are about the safety of patients, and product quality is of paramount importance to manufacturers.

Figure 3: Honeywell's 20PC Series Liquid Media Compatible Pressure Sensors offer a wide range of pressure ranges, package types, and digital/analog outputs
The reliability of the supplier is also important to the designer. Sensor manufacturers should select suppliers worldwide to support product design and production needs. For example, when European designers develop new products, they typically outsource certain modules or components to electronic manufacturing services (EMS) companies around the world, and finally assemble them at European manufacturers.
Emerging technology trends
Medical device engineers also need vendors to provide products to support emerging technology trends such as digital interface options and disposable sensors. Sensor manufacturers offer digital interfaces (especially I2C outputs) for several reasons: With digital I2C outputs, designers can remove signal conditioning circuitry on printed circuit boards (PCBs), saving board space and cost. In addition, the digital I2C output reduces the number of copper wires and wires, further reducing costs. The reduction in components has helped the board to become smaller.
Signal conditioning on the board enables compensation, calibration, and amplification of the sensor, improving measurement accuracy and reliability. This helps improve the quality of care and patient comfort and reduces the total cost of purchasing equipment in a hospital or outpatient clinic. Manufacturers of medical devices (from dialysis machines, infusion pumps to incubators, and breathing equipment) will benefit from this.
In order to save the cleaning work with the liquid medicine or the direct contact area with the patient's body fluid, more and more medical devices (such as infusion pumps) use disposable sensors. Since sensors play a decisive role in the performance of medical devices, medical personnel must consider the reliability of disposable sensors.
If the sensor needs to be replaced without replacement, cross-infection becomes a major hazard for disposable sensors. This is especially true when sensors are used to treat HIV/AIDS, avian flu and Legionnaires patients who can be infected by body fluids. Although medical device manufacturers recommend that disposable sensors be used for only one patient, some clinics do not follow this recommendation in under-budgeted and unregulated areas.
Pre:On the role of four different Hall current sensors 2025-12-30
Next:Internet of Things drives the rapid development of sensor technology 2025-12-30

 Collect
Collect
 Navigate:
Navigate: